By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Blog
Chlorine dioxide solution in metastatic cancer: case series
Written By: Manuel Aparicio-Alonso & Verónica Torres-Solórzano
Abstract
Chlorine dioxide is a potent oxidant with in vitro anticancer activity. Its precise mechanism of action has not been thoroughly explored, but it is proposed that it acts through the redox imbalance of cancer cells. Three patients were treated for metastatic cancer (kidney, prostate and lymphoma), on a compassionate basis. We report lasting tumor response with a combination of oral, enema and/or intravenous chlorine dioxide, without side effects. The patients had refused conventional chemotherapy, radiation therapy or immunotherapy. This preliminary work suggest that chlorine dioxide and his free radicals might be the mediators. Chlorine dioxide is both a promising and inexpensive anticancer agent. Rigorous clinical trials are needed to confirm these preliminary results.
Keywords: Chlorine dioxide solution, cancer, reactive oxygen species, intermittent fasting, ketogenic diet
Cite as: Manuel Aparicio-Alonso, Verónica Torres-Solórzano. Chlorine dioxide solution in metastatic cancer: case series. Authorea. July 10, 2023.
DOI: 10.22541/au.168503521.10282552/v3
Introduction
Chlorine dioxide solution (CDS) is a potent oxidant and a prodrug of HOCl, widely used as a biocide 1–3. CDS has cytotoxic effect on cancer cells4. The cytotoxicity of CDS on cancer cells appears to be associated with the induction of oxidation that disrupts the delicate and controlled redox balance of cancer cells, which, induces apoptosis, pyknosis and necrosis. Thus, CDS has the potential to prevent tissue invasion and cell transformation 5–9.The cytotoxic effect of CDS was demonstrated by inhibiting the proliferation of human cancer cell lines and pancreatic adenocarcinoma 6,7,10
CDS does not appear to be toxic to normal cells, it was shown that CDS does not have an apoptotic effect on human gingival fibroblasts and does not decrease the viability of periodontal ligament stem cells11,12. Also, in the public health context, the oral use of CDS has been reported as a safe and effective therapy to treat COVID-19 13–17.
Given the documentary evidence collected to date, we raised the possibility that a CDS may have an effective cancer treatment. We report cases of patients with metastatic cancers treated with maximum daily doses of 3 mg/kg (0.003 % chlorine dioxide) given orally, enema and/or via intravenous 13,18. For the oral and absorption enema protocol used by all patients, it was produced by oxidation of 28% sodium chlorite (NaClO2) with 4% hydrochloric acid (HCl) as the activator. The oral protocol doses were prepared with 20 ml to 30 ml of ClO2 diluted in 1L of H2O and the absorption enema protocol doses were prepared with 20 ml to 40 ml of ClO2 diluted in 500 ml to 1000 ml of H2O which was introduced with a nelaton rectal catheter to 30-50 cm 4,17. For the intravenous use, chlorine dioxide (ClO2) was made and marketed by certificate chemical expert in Queretaro, México, and was produced by the membrane electrolysis method; the dose was prepared with 10-20 ml of ClO2 diluted in 500 ml of NaCl 0.9 % and administered from 4 to 8 h according to the patient’s tolerance 17,19. The occurrence of adverse events and the manifestation of side effects that could be associated with the use of CDS were assessed.
Case presentation 1: Metastatic prostate cancer.
In October 2019, a 64-year-old Mexican male patient with no relevant medical history attended a routine prostate examination and revealed abnormal prostate antigen values (> 1700 ng/ml). Urinary and semen bleeding occurred promptly, and was diagnosed with metastatic prostate cancer. In February 2020, the patient refused chemotherapy and chose a metabolic therapy that consisted for 2.5 months of daily intravenous administration of the glucose analog 2-deoxy-D-glucose (2DG). Additionally, the patient followed a ketogenic diet and 20 h intermittent fasting. During therapy, the patient not had significant side effects.
In March 2020, the patient started the oral CDS protocol adding 1 ml of the vehicle DMSO 70% to solution and CDS absorption enema protocol. In 2021, the patient added 5 g of clinoptilolite zeolite to the diet, during fasting and before each meal. In 2022, the patient balanced oral and enema therapy with the intravenous CDS protocol, which the patient administered monthly according to previously described doses. Currently, the patient is without deficits or alterations in the daily routine. The patient has normal prostate antigen values and maintains a periodic control, with a forty-four-month follow-up (Fig. 1).
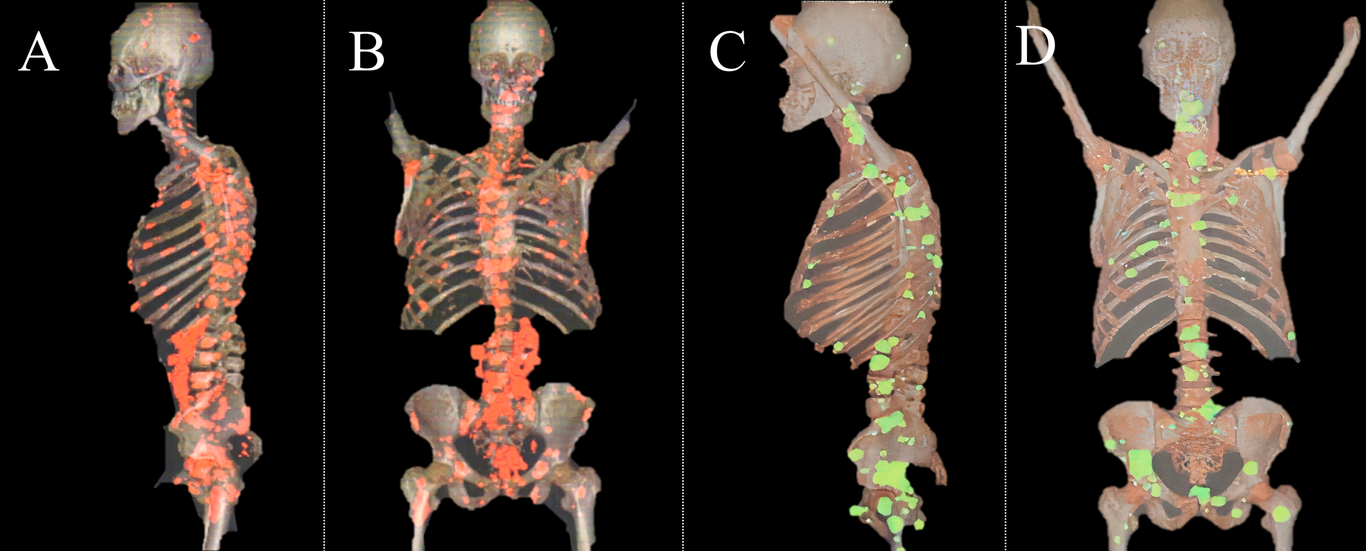
FIG 1. Positron emission tomography of a male patient (Case 1) with metastastic prostate cancer. Images confirmative the diadnosis of metastic cancer, 2019 (A,B). Images after CDS treatment, 2022 (C,D), important reduction in bone metastasis is observerd.
Case presentation 2: Metastatic kidney cancer.
In July 2018, a 65-year-old male Mexican patient with no previous medical history, but with type II diabetes, presented hematuria. A diagnosis of clear cell renal cell carcinoma was diagnosed and was tread with nephrectomy. In December 2018, at routine follow-up, two 4 mm and 5 mm (Fig. 2) lung nodules were detected, and the patient started targeted therapy with Axitinib 5 mg every 12 h and monthly immunotherapy with Pembrolizumab 100 mg/ 4 ml. The patient presented side effects such as constipation, indigestion, tinnitus, extreme fatigue, thrombocytopenia, cough, arthralgia, weight loss, rash and dysgeusia, a medical reason for intermittent discontinuation of treatment. In 2019, the size of one of the pulmonary nodules increased to 9 mm and 12 mm (Fig.3).
In December 2020, the patient decided to discontinue conventional therapy, and started the oral and absorption enema CDS protocol. Additionally, the patient practices a reduced protein diet. The patient manifested no side effects with CDS intake, currently continues the treatment and leads a normal life. As of 2023 the patient is in complete remission, with a fifty-eight-month follow-up.
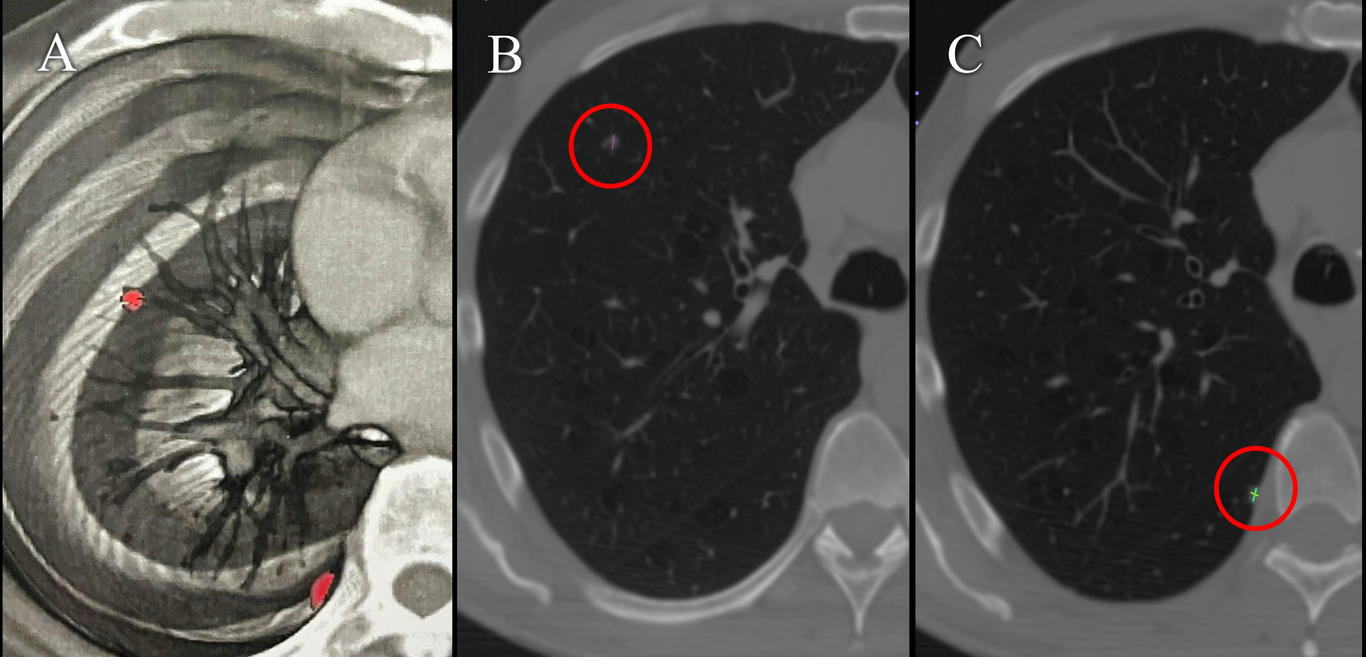
FIG 2. Positron emission tomography of a male patient (Case 2) with metastatic kidney cancer. In 2018, cancer cell activity was diagnosed in two nodules of the left lung (A) and the confirmative dimensions of 4 mm (B) and 5 mm (C).
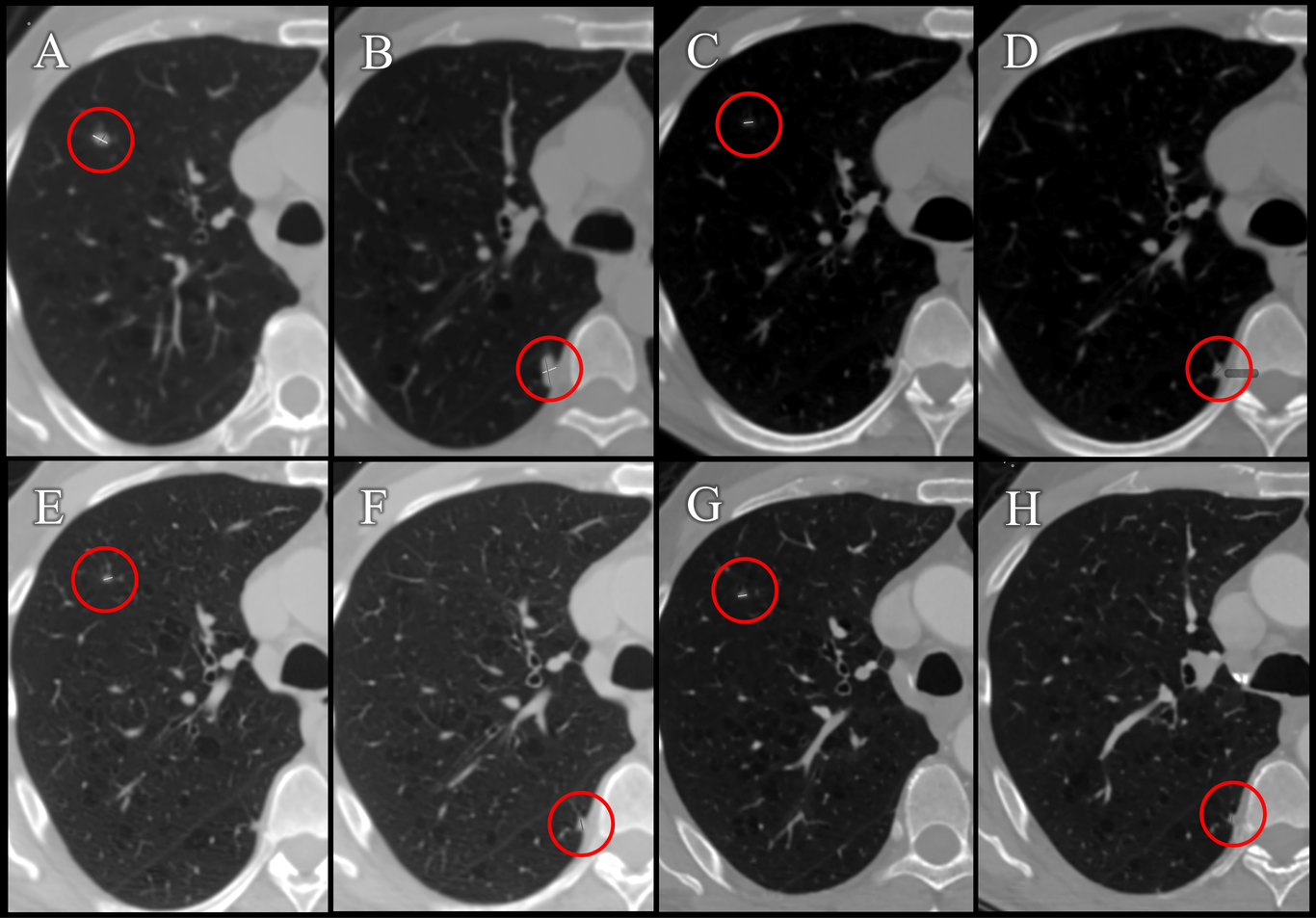
Fig. 3. PET control studies (Case 2). Monitoring pulmonary nodules in axial views of the left lung. In 2019, during chemotherapy, an increase in nodules to 9 mm (A) and 12 mm (B) was observed. In the year 2020, during compassionate treatment with CDS, the nodules reduced in size to 3.5 mm (C) and 4.7 mm (D). In the year 2021, in the routine follow-up, a maintenance in the size of the nodules of 3.4 mm (E) and a decrease to 4.2 mm (F) is observed. In the year 2023, the patient continued compassionate treatment and the nodules kept a size of 3.4 mm (G) and reduced to 3.7 mm (H).
Case presentation 3: Metastatic non-Hodgkin’s lymphoma.
In September 2019, a 73-year-old Mexican female patient, with no relevant medical history and a family history of cancer, presented incapacitating low back pain and the presence of an inflamed lymph node in the groin was found. The presence of grade 2 follicular lymphoma was confirmed by biopsy and it was diagnosticated as non-Hodgkin’s lymphoma stage IV accorded to Lugano classification. In November 2019, the patient received eight chemotherapy sessions consisted Doxorubicin 2mg/ml every 21 days, additional with ketogenic diet. The patient reported nausea, vomiting, hair loss, weakness, weight loss and dry skin as side effects. After the chemotherapy sessions, the patient had bone metastasis and refused future sessions of chemotherapy and subsequent radiation therapy.
In December 2020, the patient decided to start the CDS oral protocol and 1 ml of the vehicle DMSO 70% to solution. In 2021, when the tumor did not respond, the patient had important lower back pain, due to pathological L3 fracture, a lumbar brace was prescribed for 3 months and CDS absorption enema protocol was added. Additionally, the patient practices intermittent fasting for 18-20 hours and consumes daily supplements at night of 5,000 IU vitamin D3, 1 g vitamin C, 1.1 g potassium and 250 mg magnesium. The patient continues a thirty-eight-month follow-up, in which a significant reduction of the tumors in the invaded tissues was observed (Fig. 4), currently, without metastatic activity (Fig. 5). The patient is in partial remission.
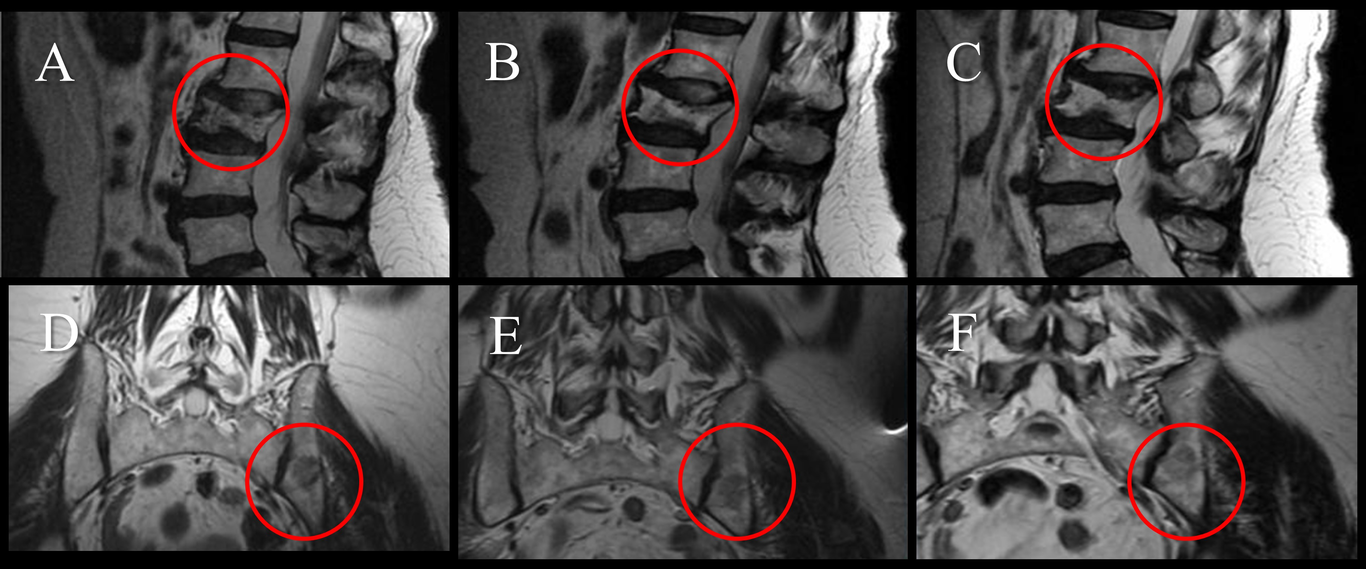
Fig. 4. Magnetic Resonance Imaging (MRI) of female patient with metastatic non-Hodgkin’s lymphoma (Case 3). Images in sagittal view showed pathological fracture in the L3 vertebra, 2020 (A), and the resolution from 2021 (B) and 2022 (C). Images showed decreased metastatic activity in both L3 and iliac bone from 2020 (D), 2021 (E) and 2022 (F).
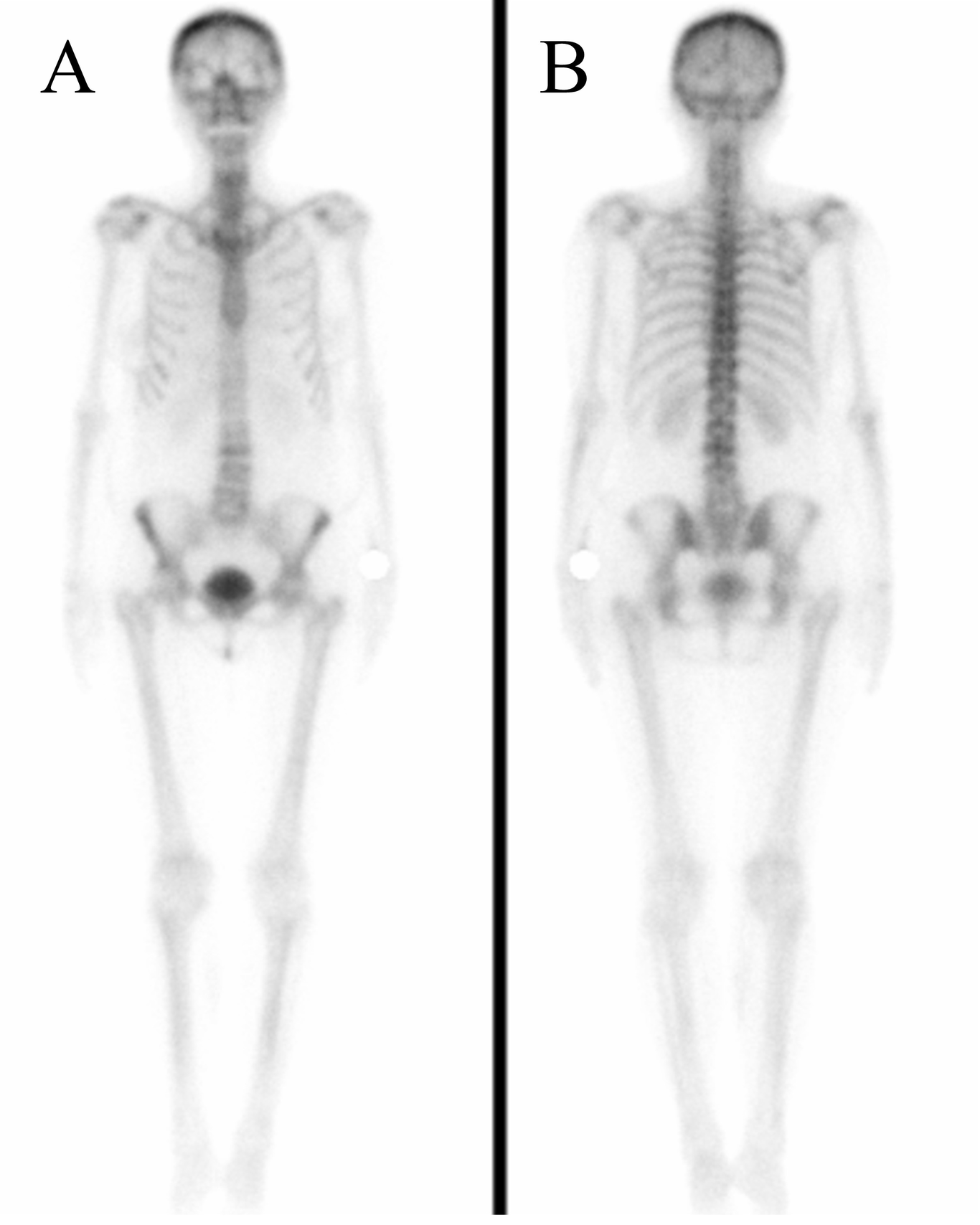
Fig. 5. Scintigraphy of female patient with metastatic non-Hodgkin’s lymphoma (Case 3). Images of ventral (A) and dorsal (B) view showed metastatic inactivity in bone from 2023. No scans from previous years.
Discussion
Chlorine dioxide therapies require controlled animal studies to evaluate posology and via of administration. The question of long-term efficacy and toxicity in patients on anticancer therapy is open. These cases suggest a broad spectrum of activity and lack of toxicity. In this article we report the use of a compassionate therapy based on chlorine dioxide solution (CDS) as part of the treatment of four clinical cases with metastatic cancer.
The first patient with metastatic prostate cancer started his therapy with the temporary administration of 2DG. The 2DG is a non-metabolizable glucose analog in transformed cells, which interferes with glycolysis and leads to the expression of stress-related genes, which subsequently trigger apoptosis 20,21. Treatment continued with the oral CDS in combination of DMSO. DMSO has anti-inflammatory, analgesic and membrane penetrating effects, its primary use is as a vehicle for other co-administered agents 22,23. Oral therapy was supplemented with enemas and intravenous administration of CDS. The rectal administration has a local as well as a systemic effect. Rectal administration has been described as a stable route, due to gastric pH elution and hepatic first pass 24,25. Also, possible systemic absorption via lymph nodes has been reported 26. Likewise, the therapy was supplemented with intravenous chlorine dioxide therapy, due to full availability in the bloodstream 4. We suggest that, multiple administration routes increased the range of action of chlorine dioxide throughout the system. CDS therapy, was supplemented with the clinoptilolite zeolite, for which has been reported to have an anticancer effect 27–30. This suggests that, possibly the induction of multiple redox changes has a relevant role in destabilizing the intracellular environment of cancer cells, thus, interfering with the Warburg effect phenotype. Additionally, intermittent fasting, a type of caloric restriction without malnutrition, was carried, which promotes anticancer adaptations 31,32. This suggests that the combination of chlorine dioxide with other therapeutic agents exhibits a synergistic anticancer effect
The second patient had metastatic kidney cancer, he decided to stop initial treatment and started a second-line oral and enema CDS therapy. As chemotherapy and immunotherapy were discontinued, the reduction in lung nodule size appears to be a direct consequence of CDS administration.
The third patient presented a metastatic non-Hodking lymphoma that was treated initially with chemotherapy sessions and continued the second-line therapy with CDS, administered systemically in combination with DMSO. In this case, the treatment was complemented with dietary supplements of vitamin D3, vitamin C, potassium and magnesium, correlated with in vitro and in vivo anticancer effects. First, low serum levels of vitamin D3 have been associated with carcinogenic tumor incidence and mortality 33–36. Also, vitamin C has been reported in preclinical studies to induce a redox imbalance and in combination with potassium to exhibit a synergistic effect on apoptosis in breast cancer cell lines 37,38. Similarly, magnesium supplementation has been explored to exert antitumor effects, such as inhibition of tumor growth in the primary site 39. However, controlled clinical studies are required to clarify the role of supplementation in metastatic cancer and the facilitation of tumor implantation.
Conclusion
For each case, after treatment with CDS in three different types of cancer, a significant antitumor response was observed in all metastatic tumors., with no associated side effects. The treatment based on chlorine dioxide is safe and cost-effective. Controlled clinical studies in patients with incurable advanced cancer are proposed to determine the efficacy and safety of CDS protocols.
Conflict of interest: None
M. Aparicio-Alonso treated patients. L. Schwartz helped write the article. V. Torres-Solórzano wrote the draft of the article. All authors contributed to the discussion of the results.
Key Clinical Message
This case series of patients with metastatic cancer treated with chlorine dioxide-based therapy highlights the potential of this treatment modality in controlling disease progression and reducing tumor burden. In addition, an improvement in cancer-related symptoms was observed, contributing to increased functionality and overall well-being of the patients.
References
1. O Young R. Chlorine Dioxide (CLO2) As a Non-Toxic Antimicrobial Agent for Virus, Bacteria and Yeast (Candida Albicans). International Journal of Vaccines & Vaccination. 2016;2(6). doi:10.15406/ijvv.2016.02.00052
2. Huang J, Wang L, Ren N, Ma F, Juli. Disinfection effect of chlorine dioxide on bacteria in water. Water Res. 1997;31(3):607-613. doi:10.1016/S0043-1354(96)00275-8
3. Ogata N. Inactivation of influenza virus haemagglutinin by chlorine dioxide: oxidation of the conserved tryptophan 153 residue in the receptor-binding site. Journal of General Virology. 2012;93(12):2558-2563. doi:10.1099/vir.0.044263-0
4. Ma JW, Huang BS, Hsu CW, et al. Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. Int J Environ Res Public Health. 2017;14(3):329. doi:10.3390/ijerph14030329
5. Ogata N. Denaturation of Protein by Chlorine Dioxide: Oxidative Modification of Tryptophan and Tyrosine Residues. Biochemistry. 2007;46(16):4898-4911. doi:10.1021/bi061827u
6. Kim Y, Kumar S, Cheon W, et al. Anticancer and Antiviral Activity of Chlorine Dioxide by Its Induction of the Reactive Oxygen Species. J Appl Biol Chem. 2016;59(1):31-36. doi:10.3839/jabc.2016.007
7. Yıldız SZ, Bilir C, Eskiler GG, Bilir F. The Anticancer Potential of Chlorine Dioxide in Small-Cell Lung Cancer Cells. Cureus. Published online October 6, 2022. doi:10.7759/cureus.29989
8. Svenson D, Kadla J, Chang H min, Jameel H. Effect of pH on the Inorganic Species Involved in a Chlorine Dioxide Reaction System. Ind Eng Chem Res. 2002;41.
9. Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8(6):385-387. doi:10.1016/S1353-8020(02)00018-4
10. Schwartz L. Chlorine dioxide as a possible adjunct to metabolic treatment. J Cancer Treatment Diagn. 2017;1(1):6-10. doi:10.29245/2578-2967/2018/1.1107
11. Nishikiori R, Nomura Y, Sawajiri M, Masuki K, Hirata I, Okazaki M. Influence of chlorine dioxide on cell death and cell cycle of human gingival fibroblasts. J Dent. 2008;36(12):993-998. doi:10.1016/j.jdent.2008.08.006
12. Láng O, Nagy KS, Láng J, et al. Comparative study of hyperpure chlorine dioxide with two other irrigants regarding the viability of periodontal ligament stem cells. Clin Oral Investig. 2021;25(5):2981-2992. doi:10.1007/s00784-020-03618-5
13. Insignares-Carrione E, Bolano Gómez B, Andrade Y, et al. Determination of the Effectiveness of Chlorine Dioxide in the Treatment of COVID 19. Journal of Molecular and Genetic Medicine. 2021;15.
14. Mitchell BL. The chlorine dioxide controversy: A deadly poison or a cure for COVID-19? International Journal of Medicine and Medical Sciences. 2021;13(2):13-21. doi:10.5897/IJMMS2021.1461
15. Aparicio-Alonso M, Domínguez-Sánchez C, Banuet-Martínez M. COVID19 Long Term Effects in Patients Treated with Chlorine Dioxide. International Journal of Multidisciplinary Research and Analysis. 2021;04(08). doi:10.47191/ijmra/v4-i8-14
16. Aparicio-Alonso M, Domínguez-Sánchez C, Banuet-Martínez M. A Retrospective Observational Study of Chlorine Dioxide Effectiveness to Covid19-like Symptoms Prophylaxis in Relatives Living with COVID19 Patients. International Journal of Multidisciplinary Research and Analysis. 2021;04(08). doi:10.47191/ijmra/v4-i8-02
17. Aparicio-Alonso M, Domínguez-Sánchez C, Banuet-Martínez M. Determination of the Effectiveness of Oral Chlorine Dioxide in the Treatment of COVID 19. Journal of Infectious Diseases & Therapy. Published online 2021.
18. Environmental Protection Agency. Toxicological Review of Chlorine Dioxide and Chlorite. CAS Nos. 10049-04-4 and 7758-19-2. In Support of Summary Information on the Integrated Risk Information System.; 2000.
19. Kály-Kullai K, Wittmann M, Noszticzius Z, Rosivall L. Can chlorine dioxide prevent the spreading of coronavirus or other viral infections? Medical hypotheses. Physiol Int. 2020;107(1):1-11. doi:10.1556/2060.2020.00015
20. Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87(7):805-812. doi:10.1038/sj.bjc.6600547
21. Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355(2):176-183. doi:10.1016/j.canlet.2014.09.003
22. Aronson. Dimethylsulfoxide. In: Meyler’s Side Effects of Drugs. Elsevier; 2016:992-993. doi:10.1016/B978-0-444-53717-1.00633-8
23. Gad SE, Sullivan DW. Dimethyl Sulfoxide (DMSO). In: Encyclopedia of Toxicology. Elsevier; 2014:166-168. doi:10.1016/B978-0-12-386454-3.00839-3
24. Davis MP, Walsh D, LeGrand SB, Naughton M. Symptom control in cancer patients: the clinical pharmacology and therapeutic role of suppositories and rectal suspensions. Supportive Care in Cancer. 2002;10(2):117-138. doi:10.1007/s00520-001-0311-6
25. Hua S. Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Front Pharmacol. 2019;10. doi:10.3389/fphar.2019.01196
26. Purohit TJ, Hanning SM, Wu Z. Advances in rectal drug delivery systems. Pharm Dev Technol. 2018;23(10):942-952. doi:10.1080/10837450.2018.1484766
27. Pavelić K, Hadžija M, Bedrica L, et al. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med. 2001;78(12):708-720. doi:10.1007/s001090000176
28. Katic M. A clinoptilolite effect on cell media and the consequent effects on tumor cells in vitro. Frontiers in Bioscience. 2006;11(1):1722. doi:10.2741/1918
29. DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106-109. doi:10.1038/nature10189
30. Ryoo I geun, Lee S hwan, Kwak MK. Redox Modulating NRF2: A Potential Mediator of Cancer Stem Cell Resistance. Oxid Med Cell Longev. 2016;2016:1-14. doi:10.1155/2016/2428153
31. Clifton KK, Ma CX, Fontana L, Peterson LL. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J Clin. 2021;71(6):527-546. doi:10.3322/caac.21694
32. Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89-98. doi:10.1016/j.tips.2009.11.004
33. Park HY, Hong YC, Lee K, Koh J. Vitamin D status and risk of non-Hodgkin lymphoma: An updated meta-analysis. PLoS One. 2019;14(4):e0216284. doi:10.1371/journal.pone.0216284
34. Grant WB, Juzeniene A, Moan JE. Review Article: Health benefit of increased serum 25(OH)D levels from oral intake and ultraviolet-B irradiance in the Nordic countries. Scand J Public Health. 2011;39(1):70-78. doi:10.1177/1403494810382473
35. Vuolo L, di Somma C, Faggiano A, Colao A. Vitamin D and Cancer. Front Endocrinol (Lausanne). 2012;3. doi:10.3389/fendo.2012.00058
36. Grant WB, Mohr SB. Ecological Studies Of Ultraviolet B, Vitamin D And Cancer Since 2000. Ann Epidemiol. 2009;19(7):446-454. doi:10.1016/j.annepidem.2008.12.014
37. Frajese G, Benvenuto M, Fantini M, et al. Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Oncol Lett. 2016;11(6):4224-4234. doi:10.3892/ol.2016.4506
38. Ngo B, van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19(5):271-282. doi:10.1038/s41568-019-0135-7
39. Barbagallo M, Veronese N, Dominguez LJ. Magnesium in Aging, Health and Diseases. Nutrients. 2021;13(2):463. doi:10.3390/nu13020463
Source: https://www.authorea.com/users/568645/articles/643826-chlorine-dioxide-solution-in-metastatic-cancer-case-series
